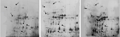Research Article
Screening and Expression Analysis of Temperature-responsing Proteins that Can Remove Low Temperature Stagnancy of Seeds Germination of Lepidium apetalum 
2 Beijing Key Laboratory of Gene Resource and Molecular Development, College of Life Science, Beijing Normal University, Beijing, China, 100875
 Author
Author  Correspondence author
Correspondence author
Molecular Plant Breeding, 2016, Vol. 7, No. 18 doi: 10.5376/mpb.2016.07.0018
Received: 29 Mar., 2016 Accepted: 03 May, 2016 Published: 12 May, 2016
Zhao J.J., Zhao H.X., Li P.P., Zeng W.J., Li Y.H., Ge F.W., Zhu C.Q., Lu H., and Zhao H.P., 2016, Screening and Expression Analysis of Temperature-responsing Proteins that Can Remove Low Temperature Stagnancy of Seeds Germination of Lepidium apetalum, Molecular Plant Breeding, 7(18): 1-8 (doi: 10.5376/mpb.2016.07.0018)
As a kind of ephemeral plant, Lepidium apetalum Willd. in northern XinJiang shows special responsible characteristics to temperature during the germination: low temperature stratification could improve germination rate and homogeneity of L.apetalum seeds. But it could not germinate and be at stagnation stage under 4°C, while this phenomenon could be removed by the treatment with 25°C for 45 minutes or longer time. The mechanism why the treatment by higher temperature for a short time could remove germination stagnation under low temperature is not clear yet. In this study, the total proteins difference of seeds with three treatments were analyzed by2-DE, which the seeds were without stratification, stayed at germination stagnation stage under low temperature and removed stagnation with 25℃treatment. Screened proteins responsing to temperature which were related to remove germination stagnation under low temperature, and the corresponding genes expression were confirmed by qRT-PCR. The result showed that there was no obvious difference between the seeds without stratification and the seeds at germination stagnation stage by low temperature. But the proteins expression was quite different between the seeds treated with 25°C for 45 minutes to remove the germination stagnation and the other two treated samples. 37 distinct protein spots were found in the 2-DE map with 14 up-regulated spots, 23 down-regulated spots. Among them, 6 up-regulated proteins of 14 spots were identified by LC-MS/MS, CDC48E, HSP17.6 and PER12 were further chosen to be confirmed expression analysis by qRT-PCR.The result showed that their relative expression levels were significantly higher in seeds removed stagnation than in seeds before breaking stagnation. In addition, their expression levels were obviously increased with the time prolonging treated by 25°C with the highest level with the dealt for 45-55 min and then maintaining this level of expression. The result showed that the genes expression levels were positively correlated to the germination percentage treated with 25°C from 30-60 minutes. All the above suggested that Cdc48E, Hsp17.6, and Per12 induced by 25°C might be related to the germination stagnation removing of the seeds of L.apetalum. This conclusion provides a new clue for the study on the signal response to temperature in plants.
Introduction
Lepidium apetalum Willd., an annual herbaceous ephemeralsis of Cruciferae Lepidium, its root, stem and leaf have large parenchyma cells (Yu and Liu, 1997), and its vascular bundle possess a characteristic anatomy called kranz anatomy with high light effect, belongs to C4 plants (Li, 2009). L. apetalum seeds are flat egg shaped and in reddish brown, its rich in fat, protein, sugar, mustard glycosides, and have effect of cardiac, antiasthmatic, diuretic. Similar to Lepidium perfoliatum seeds, L. apetalum seeds’ dry surface covered with a layer of mucus quality seed coating, which main component is polysaccharide. In case of water, the seed coating emit outside like rays (Gu, 2008), and absorb moisture quickly from the surrounding soil for germination, but it barely work on seedling growth (Yuan et al., 2006). The L. apetalum live in the desert of Northern Xinjiang, is pioneer plant in flora (Li, 2014), so it has important ecological significance. And the L. apetalum has the characteristics of typical ephemeral plants, which can tolerant low temperature to germinate and grow in early spring. Zhao Huixin et al.,(2012) screened and acquired 18 genes of cold up-regulated expression genes from L. apetalum seedlings using cDNA-AFLP technology, this work provides some clues for the study of the molecular basis of L. apetalum seedling tolerance to low temperature stress.
L. apetalum shows special responsible characteristics to temperature during the germination. In laboratory, L. apetalum seeds cannot germinate at low temperature (0°C-4°C), but germinate at 10°C to 25°C (Meng et al., 2008). However, in the field, L. apetalum seedscould germinate at 2°C-3°C, the average temperature of early spring in Northern Xinjiang, it shows a good tolerance to low temperature stress. Why L. apetalum seeds can germinate at low temperature in early spring but cannot germinate at low temperature in the laboratory? The study of Zhao Huixin (2010) pointed out that, although L. apetalum seeds cannot germinate at 4°C in the laboratory, it can tolerate low temperature before and after a critical physiological stage, the stage before seeds just sprout. If the temperature is low in early spring, there will be a stagnancy stage in early germination of L. apetalum seeds after it imbibes moisture, but this stagnancy will be broken if there is a certain time of rise in the temperature. It is consistent with the experimental results that appropriate stratification at low temperature in laboratory can significantly improve germination energy of L. apetalum seeds, and the result that L. apetalum seeds can germinate at 4°C only after be treated for 1 h at 25°C after 10 d’s stratification. In addition, L. apetalum seeds treated by cold induction in various periods over low temperature stagnancy has stronger tolerance to -5°C, -10°C stress (Meng et al., 2008). How can L. apetalum seeds tolerate low temperature to germinate? To analyze the physiological mechanism of this, Yang Na (2015) have studied and reported that, in the process of L. apetalum seeds germination, low temperature had a great effect on the activity of superoxide dismutase (SOD) and catalase (CAT). Zhou Qian (2016) sequenced the transcriptome of L. apetalum seeds before and after germination stagnancy at low temperature, the result showed that 159 genes had significant differences in expression, and 54 genes expression was up-regulated significantly. While the molecular mechanism of L. apetalum seeds tolerate low temperature to germinate has not been reported at protein level. This study screened proteins responding to temperature related to germination stagnancy and removement of germination stagnancy, then identified important differential proteins, finally, discussed these proteins’ function, gene expression patterns in the process of removing germination stagnancy, and analyzed the correlation between their expression quantity and germination stagnancy, to lay the basis for clarifying the mechanism of L. apetalum seeds germination stagnancy at low temperature.
1 Results
1.1Changes in total protein during germination of L. apetalum seeds
Total protein was extracted respectively from three groups of L. apetalum seeds with TCA-Acetone precipitation methods, and protein content was measured respectively. The results showed, the total protein content in the first group of seeds (The seeds without stratification) has reached to 3.46%, was the highest in three groups, which may contain a large amount of storage protein. The total protein content in the second group of seeds (The seeds stayed at stage of germination stagnancy after stratification for 10 d at low temperature) has decreased to 2.91% significantly. May be in the initial germination period, a large number of storage protein was decomposed to provide energy for germination or changed into other non-protein substances for the need of germination. The total protein content in the third group of seeds (The seeds removed stagnancy after treated for 50 min at 25℃)has reached to 3.19%, was significantly increased than the second group. It showed that there were some new proteins synthesized in the process of removing germination stagnancy, which may be closely related to the germination of L. apetalum seeds at low temperature.
1.2 Screening for temperature-responding proteins during germination of L. apetalum seeds
There were about 600 protein spots were separated by two-dimensional gel electrophoresis (2-DE) in every groups of seeds. We found 37 distinct protein spots (Figure 1) in the 2-DE map of the third group different from the other two groups, of which 14 of them were up regulated and 23 down regulated. 6 up-regulated proteins (24, 27, 28, 29, 31, 32) were identified by LC-MS/MS, and the result was analyzed in Swissprot and NCBI database with MASCOT. These 6 proteins is molecule chaperon protein Cpn60, heat shoct proteins Hsp17.6I and Hsp70B, cell division control protein 48 homolog E (Cdc48E), peroxidase 12 (Per12) and peroxidase 28 (Per28) of oxidoreductase family Ⅲ respectively. As a molecular chaperone, Cpn60 involved in protein folding, and help related proteins play a corresponding role. When the seed subjected to heat shock, osmotic shock or salt stress in the process of development and germination, Hsp17.6I will express and localize in cytoplasm, it also plays the role of molecular chaperone (Sun et al., 2001). After a brief heat shock to L. apetalum seeds, heat shock protein will synthesized in response to environmental changes. Cell division control protein 48 homolog E is a member of the cell division control protein family, it participates in the regulation of cell division, and may be involved in cell division and proliferation during germination of seeds. Peroxidase (POD) has a relationship with respiration, photosynthesis and auxin oxidatio, its physiological activity varied with the processes of plant growth and development, and it may be involved ingermination of L. apetalum seeds at low temperature. In subsequent experiments, the expression of molecular chaperone Hsp17.6, peroxidase Per12 and cell division control protein Cdc48E were further confirmed.
|
Figure 1 Comparison of 2-DE maps of total seed protein before and after germination stagnation under low temperature |
1.3 Analysis of the relationship between the protein expression of L. apetalum seeds response to low temperature and germination at low temperature
1.3.1 Analysis of the expression of CDC48E, PER12, and HSP17.6 genes before and after removing germination stagnancy of L. apetalum seeds
To further explore the effect of protein expression on the removing germination stagnant of L. apetalum seeds, we compared the expression of CDC48E, PER12 and HSP17.6 genes of three groups of seeds in this study. The results showed, these genes expressed very low in both the first group and the second group, but was significantly up-regulated in the third group. It descript that, short thermal stimulation may promote the expression of CDC48E, PER12 and HSP17.6 genes, and the expression of these genes may closely associated with removing germination stagnancy of L. apetalum seeds (Figure 2).
|
Figure 2 The genes expression analysis of Lepidium apetalum Willd before and after germination stagnation under low temperature |
1.3.2 Analysis of the relationship between the protein expression of CDC48E, PER12 and HSP17.6 and low temperature germination
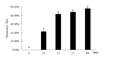
L. apetalum seeds, in the germination period of stagnancy after stratified for 10 d at 4°C, was treated for 0 min, 30 min, 45 min, 55 min and 60 min at 25°C, then the germination rate of it tolerate to low temperature was counted(Figure 3). After induction treatment, the germination rate of L. apetalum seeds was 0%, 40%, 83.33%, 88.33%, and 96.11% respectively in 4°C conditions. That is to say, 40% of seeds can remove the germination stagnancy and germinate at low temperature after treated for 30 minutes at 25°C; after treated for 45 minutes at 25°C, 83.33% of the seeds remove the germination stagnancy; and after treated for 60 minutes at 25°C, the germination rate of seeds tolerance to low temperature reached germination rate at normal temperature, which is almost all the seeds can remove the germination stagnancy.
|
Figure 3 Germination rates of the Lepidium seeds under low temperature after dealing with 25℃ for different time |
To further explore the relationship between the expression of screened-related protein in responds to low temperature and germination of L. apetalum seeds, the expression of CDC48E, PER12 and HSP17.6 genes were analyzed, after the seeds of low-temperature germination stagnancy were induced by different time of treatment at 25°C (Figure 4). Compared with the L. apetalum seeds of low temperature stagnancy, the expression of CDC48E, PER12 and HSP17.6 genes was extremely up-regulated (p<0.01) after treated for 30 min at 25°C, and the expression showed a rising trend along with the increase of the treatment time within 60 min. Expression characteristics of these three genes also have some differences. PER12, CDC48E gene were extremely up-regulated for different time at 25°C, while the expression of HSP17.6 gene was extremely up-regulated in the treatment of 50-60 min at 25°C (p<0.01), and significantly up-regulated at 25°C, 45 min (p<0.05). The result is consistent with the trend of that, more than 80% of the seeds can remove the germination stagnancy and germinate at low temperature after treated for 45 min at 25°C. It also suggests that the proteins which expression was up-regulation at 25°C closely related to remove the germination stagnancy of L. apetalum seeds at low temperature.
|
Figure 4 The genes expression analysis at the different germination stage of Lepidium apetalum Willd |
2 Discussion
Seed germination is a very complex process, which involves the regulation and expression of many proteins associated with the growth and development. In this study, we choose L. apetalum seeds of the special ecotype live in northern Xinjiang as research material. By comparing the different expression of protein before and after removing the low-temperature germination stagnancy, the significantly up-regulated proteins of seeds, which low-temperature germination stagnancy was removed, were screened and identified by mass analysis. Then, the expression analysis of corresponding gene was performed for some of these proteins.
Heat shock protein is a kind of highly conservative molecule chaperones in the process of system development. It can be divided into Hsp100s, Hsp90s, Hsp70s, Hsp60s and sHsps, according to molecular weight (KD). The first four Hsp is considered HMW Hsps (high molecular weight Hsps), they are mainly involved in protein folding and assembly. Hsp70B located in the cytoplasm membrane, chloroplast and cytoplasm, it participates in the enrichment and folding of nascent protein that thermally induced synthesis in cytosol and organelles associates with other molecular chaperones, it also can reduce membrane damage of plant in cold environment, enhance the cold resistance of organization, and closely associated with plant acquired tolerance (Lin et al., 2001). Huang Shangzhi et al., (2004) found that the cold resistance of the rice seedlings was significantly enhanced if the seeds were treated by 42°C heat shock during germination. Molecular chaperone Cpn60-α1 subunits in cytoplasm can combine Rubisco large subunit (Chen, 2000) to help protein correctly folding and free from the interference of other proteins. The expression of Hsp17.6I was increased in the process of L. apetalum seeds relieving low-temperature stagnancy, it improve the cold tolerance of L. apetalum.
Peroxidase (POD) is a protective enzyme within plant cells, it is related to the plants resistance to low temperature and other adverse environment. In a certain range of temperature, the POD activity increase with the increase of temperature (Liu, 2001). Per12 and Per28 are typical members of oxidoreductase family Ш (Tognolli et al., 2002), they belongs to the transmembrane protein, and mainly exist in the vacuole. Their expressions are induced by the environment in the early development of seed, and they have many cofactor bindings to enzyme, such as Heme b and Ca2+binding sites (Theologis et al., 2000; Dunand et al., 2002). When plant tissues are subjected to environmental stresses such as trauma, pathogen invasion, and oxidative stress, they can remove H2O2, a toxic reducing agent, rely on various isozyme subtypes; synthetize and degradation lignin, cork, and metabolise auxin (Schenk et al., 2000). The peroxidase content increases in L. apetalum seeds which are stimulated by short-term high temperature in low temperature stratification period, it is conducive to remove H2O2, and improve L. apetalum tolerance to low temperature. However, inhibiting the expression of certain peroxidase genes such as ascorbate peroxidase (APX) (Sung et al., 2005) during the early germination, H2O2 can be accumulated to stimulate seed germination and improve the germination rate. Therefore, although the increase of the peroxide in the process of L. apetalum seeds removing germination stagnancy can protect L. apetalum from low temperature damage, but it not the key gene to promote the germination.
Cell division control protein E (Cdc48E) belongs to the family of AAAATPase, it mainly located in the nucleus. In the process of cell division, Cdc48E positioned on a film body along with the formation of the new cell wall, and play a role in the process of cell division and growth by fusion with the same type of organelles (Tabata et al., 2000; Seki et al., 2002). Cdc48E typically contains 809 amino acids, it highly expressed in the vigorous growth of cells such as bud of Arabidopsis thaliana, and it participated in the formation of new cell wall, as well as the disintegration and reconstruction of the nuclear envelope (Yamada et al., 2003; Saracco et al., 2009). After the L. apetalum seeds stratificated at low temperature for 10 d, was treated at 25°C for 1 hour, Cdc48E protein content increased significantly in the seeds, it promotes the cell cycle and is beneficial to the further growth and development of embryonic cells.
In this study, gene expression of L. apetalum seeds in different periods of low-temperature germinate was analyzed and discussed from transcription level and protein level respectively. Results showed that the expression of cell division control protein E gene (CDC48E), peroxidase 12 genes (PER12) and small heat shock protein gene (HSP17.6) were significantly up-regulated in L. apetalum seeds, the seeds of low-temperature stratification and was treated in 25°C for a period of time. This is consistent with the results of protein expression. The germination rate of L. apetalum seeds in germination stagnancy was affected by the length of 25°C treatment, and it increased significantly with the increase of treatment time. The phenotypic results also corroborated that short treatment at 25°C will affect the expression of certain genes of L. apetalum seeds in low temperature stratification, and the products of these genes expression will exert beneficial effects on L. apetalum seeds germination at low temperature. Low temperature is one of the key adverse factors that affect the yield of crops. Research on the key factor of L. apetalum seeds germination at low temperaturecan help to understand the molecular mechanism of low-temperature germination, and ultimately provides the basis for modifying crop genetic shape.
3 Materials and Methods
3.1 Materials and treatment
L. apetalum seeds were collected in LiYv hill, Urumqi, Xinjiang. Plump mature seed was selected and stored at room temperature.
The effects of different temperature treatments on removing the stagnancy of low-temperature germination were compared, the effect of 25°C was the best (Zhao, 2010). The treatment of seeds were divided into three groups: The first group is the seeds before low-temperature stratification, that is, the seed is directly wetted with sterile water; The second group is the seeds in the low-temperature germination stagnancy, that is, the seed placed at 4°C for 10 days after be wetted; The third group is the seeds that removed the low-temperature germination stagnancy, that is, the seed placed at 25°C for 50 min after it was stratificated. The samples of seeds were ground into powder in liquid nitrogen and placed in the refrigerator at -80℃ for subsequent experiment.
3.2 Method
3.2.1 Preparation of protein sample and determination of the protein content
The total protein of three groups of seeds were extracted by TCA/ acetone precipitation method respectively (Agnieszka and Juan, 2013; Vasconcelos et al., 2005; Wu et al., 2014). The concentration of total protein was measured by Bradford method (1976), and the protein content of three samples was calculated respectively.
3.2.2 Screening for temperature-responding proteins during germination of L. apetalum seeds
The proteins of three groups are isolated with two-dimensional electrophoresis method. 17 cm precast linear IPG strips (pH3-10) were chosen for the Isoelectric Focusing, sample volume was unified according to the quality of seed. Methods refer to 2-DE process of spring soybean (Glycine max L.) (Tian et al., 2015) and wild wheat (Triticum urartu L.) (Gharechahi et al., 2014), the methods were changed slightly in this study. Compared with the first and second group, screening differentially expressed proteins in the third group of seeds which removed the low-temperature germination stagnancy. PDQuest8.0 was used to analyze the 2-DE map, protein spots which expression changed more than 3 times were screened, and identified using LC / MS-MS.
3.2.3 Analysis of the relationship between the protein expression of L. apetalum seeds response to low temperature and germination at low temperature
The expression of several proteins, response to low temperature during germination, in the three groups of seeds were compared and screened. The seeds in the low-temperature germination stagnancy were divided into six parts, and were respectively treated for 0 min, 30 min, 45 min, 50 min, 55 min, 60 min at 25°C, for analyzing and verifying the gene expression of different protein by RT - PCR. Part of seeds continue to be placed at 4°C for germination, germination rate of them was counted after 15 days. Comprehensive experimental result is to analyze the relationship between the protein expression of L. apetalum seeds response to low temperature and germination at low temperature.
The protein expression was detected by fluorescence quantitative RT-PCR, specific operations are as follows: Total RNA of L. apetalum seeds was extracted using the method of Trizol (TIANGEN). Synthesizing the first cDNA of the RNA samples refers to the operating instructions of Revert Aid First Strand cDNA Synthesis Kit (Thermo). Based on the results of MASCOT, the primers were designed by Primer5.0, according to the corresponding nucleotide sequence of the target protein. The primer sequences, annealing temperature and lengths of PCR products (Table 1). With ACTIN as the reference gene, use UltraSYBR Mixture (With ROX II) (CWBIO) for the purposes of gene expression analysis. Each sample was done with three biological repeats.
|
Table 1 Sequences of the primers used in qRT-PCR analysis for key protein and the product size |
Authors' contributions
Li Pingping and Zhao Junjie are the main executors of experimental research and data analysis of this project. Zhao Junjie is the main writer of first draft of the thesis. The thesis was guided and modified by Zhao Heping and Zhao Huixin. Zhao Heping, Zeng Weijun to carry out the modification of abstracts. Experimental design and data analysis was guided by Zeng Weijun, Zhao Heping, Li Yanhong, Ge Fengwei and Zhu Changqing. Li Pingping, Zhao Junjie, and Lu Han to participate in the process of experiment, analysis the results of experiment, and make graphs and tables based on the result. Zhao Huixin is the designer of the experiment, the designer and the person in charge of the project. All the authors have read and agreed to the final text.
Acknowledgement
This work was co-supported by a grant from the Natural Science Foundation of China (No. 31460041 ), the project of “Key Laboratory of application and regulation of species diversity in the special environment of Xinjiang”, the key Laboratory of Department of Education of Xinjiang Uygur Autonomous Region (XJTSWZ-2015-01), Key Laboratory of resistance gene resources and molecular development of Beijing City (2015GD03), and PhD Programs Intital Fountion of Xinjiang Normal University (XJNUBS1416).
Agnieszka Z., and Juan D.R., 2013, A protocol for protein extraction from lipid-rich plant tissues suitable for electrophoresis, Plant Proteomics, 1072: 85-91
Bradford M.M., 1976, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Analytical Biochemistry, 72: 248-254
Chen G.Y., Xiao Y.Z., and Li L.R., 2000, Rubisco assembly in higher plant and characterization of its intermediates, Zhiwu Shengli Xuebao (Acta Phytophysiological Sinica), 26(1): 16-22
http://dx.doi.org/10.1016/0003-2697(76)90527-3
Dunand C., Tognolli M., and Overney S., 2002, Identification and characterization of Ca (2+)-pectate binding peroxidases in Arabidopsis thaliana, Journal of Plant Physiology, 159(11): 1165-1171
http://dx.doi.org/10.1078/0176-1617-00768
Gharechahi J., Alizadeh H., Naghavi M.R., and Sharifi G., 2014, A proteomic analysis to identify cold acclimation associated proteins in wild wheat(Triticum urartu L.), Molecular Biology Reports, 41(6): 3897-3905
http://dx.doi.org/10.1007/s11033-014-3257-8 PMid:24535272
Gu L.L., Liu L.H., You T.Y., Lan H.Y., and Zhang F.C., 2008, Characterization of the seed coat mucilage properties of ephemeral plant Lepidium perfoliatum L. in Xinjiang, Xibei Zhiwu Xuebao (Acta Botanica Boreali-Occidentalia Sinica), 28(12): 245-246
Huang S.Z., Huang X.F., Lin X.D., Zhang Y.S., Liu J., and Fu J.R., 2004, Induction of chilling tolerance and heat shock protein synthesis in rice seedlings by heat shock, Zhiwu Shengli Yu Fenzi Shengwuxue Xuebao (Journal of Plant Physiology and Molecular Biology), 30(2): 189-194
Li X.M., Liu P., Gu L.L., Yao S.X., You T.Y., Lan H.Y., and Zhang F.C., 2009, Studies on the C4-related structures of two ephemerals-eremopyrum orientale(L.) and Lepidium apetalum Willd. in Xinjiang, Xinjiang Nongye Kexue (Xinjiang Agricultural Sciences), 46(1): 28-33
Li Y., Feng Y., Liu B., Lv G.H., and Wang X.Y., 2014, Phylogenetic relationships and divergence time of Brassicaceae ephemeral plants in Xinjiang, Ganhanqu Yanjiu (Arid Zone Research), 31(6): 1100-1108
Lin B.L., Wang J.S., and Liu H.C., 2001, Genomic analysis of the Hsp70 superfamily in Arabidopsis thaliana, Cell Stress & Chaperones, 6(3): 201-208
http://dx.doi.org/10.1379/1466-1268(2001)006<0201:GAOTHS>2.0.CO;2
Liu Y.D., Cao T., Xiang F., and Peng C.H., 2001, Effect of high temperature stress on the activity of peroxidase of two species of mosses, Guangxi Zhiwu (Guihaia), 21(3): 255-258
Meng J., Li Q., and Li G., 2008, Physiological characteristic of seed germination of two species of Lepidium L, Shengwu Jishu (Biotechnology), 18(2): 32-35
Saracco S.A., Hansson M., and Scalf M., 2009, Tandem affinity purification and mass spectrometric analysis of ubiquitylated proteins in Arabidopsis, Plant Journal, 59(2): 344-358
http://dx.doi.org/10.1111/j.1365-313X.2009.03862.x PMid:19292762 PMCid:PMC3639010
Schenk P.M., Kazan K., Wilson I., Jonathan P.A., Todd R., Shauna C.S., and John M.M., 2000, Coordinated plant defense responses in Arabidopsis revealed by microarray analysis, Proceedings of the National Academy of Sciences of the United States of America, 97(21): 11655-11660
http://dx.doi.org/10.1073/pnas.97.21.11655 PMid:11027363 PMCid:PMC17256
Seki M., Narusaka M., Kamiya A., Ishida J., Satou M., Sakurai T., Nakajima M., Enju A., Akiyama K., Oono Y., Muramatsu M., Hayashizaki Y., Kawai J., Carninci P., Itoh M., Ishii Y., Arakawa T., Shibata K., Shinagawa A., and Shinozaki K., 2002, Functional annotation of a full-length Arabidopsis cDNA collection, Science, 296(5565): 141-145
http://dx.doi.org/10.1126/science.1071006 PMid:11910074
Sun W., Bernard C., van de Cotte B., Montagu M.V., and Verbruggen N., 2001, At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression, Plant Journal, 27(5): 407-415
http://dx.doi.org/10.1046/j.1365-313X.2001.01107.x PMid:11576425
Sung C.M., Soon I.K., and Chung S.A., 2005, Changes in expression of the cytosolic ascorbate peroxidase gene, Ca-cAPX1, during germination and development of Hot Pepper seedlings, Journal of Plant Biology, 48(3): 276-283
http://dx.doi.org/10.1007/BF03030523
Tabata S., Kaneko T., Nakamura Y., Kotani H., Kato T., and Asamizu E., 2001, Sequence and analysis of chromosome 5 of the plant Arabidopsis thaliana, Nature, 408(6814): 823-826
Theologis A., Ecker J.R., Palm C.J., et al., 2000, Sequence and analysis of chromosome 1 of the plant Arabidopsis thaliana, Nature, 408(6814): 816-820
http://dx.doi.org/10.1038/35048500 PMid:11130712
Tian X., Liu Y., Huang Z.G., Duan H.P., Tong J.H., He X.L., Gu W.H., Ma H., and Xiao L.T., 2015, Comparative proteomic analysis of seedling leaves of cold-tolerant and -sensitive spring soybean cultivars, Molecular Biology Reports, 42(3): 581-601
http://dx.doi.org/10.1007/s11033-014-3803-4 PMid:25359310
Tognolli M., Penel C., Greppin H., and Simon P., 2002, Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana, Gene, 288(1-2): 129-138
http://dx.doi.org/10.1016/S0378-1119(02)00465-1
Vasconcelos É.A.R., Nogueira F.C.S., and Abreu E.F.M., 2005, Protein extraction from cowpea tissues for 2-D gel electrophoresis and MS analysis, Chromatographia, 62(7-8): 447-450
http://dx.doi.org/10.1365/s10337-005-0637-1
Wu X.L., Gong F.P., and Wang W., 2014, Protein extraction from plant tissues for 2DE and its application in proteomic analysis, Proteomics, 14(6): 645-658
http://dx.doi.org/10.1002/pmic.201300239 PMid:24395710
Yamada K., Lim J., Dale J.M., Chen H.M., Shinn P., Palm C.J., et al., 2003, Empirical analysis of transcriptional activity in the Arabidopsis genome, Science, 302(5646): 842-846
http://dx.doi.org/10.1126/science.1088305 PMid:14593172
Yang N., Zhao H. P., Ge F.W., Li Y.H., Zeng W.J., and Zhao H.X., 2015, Physiological response of two Lepidium species to low temperature stress during seed germination, Ganhanqu Yanjiu (Arid Zone Research), 32(4): 760-765
Yu X.F., and Liu A.Q., 1997, A anatomical study on the ephemeral plant-Lepidium Apetalum Willd. in XinJiang, Xinjiang Shifan Daxue Xuebao (Journal of Xinjiang Normal University(Natural Science Edition)), 02: 34-38
Yuan Z.Y., Duo L.K.•M.M.T.Y.S.F., and Huang P.Y., 2006, The relationship between water and the seed coat of Lepidium Apetalum- an ephemeral, Zhongzi (Seed), 25(9): 1-3
Zhao H.X., Li Q., LiG., and Du Y., 2012, cDNA-AFLP Analysis reveals differential gene expression in response to cold stress in Lepidium apetalum during seedling emergence, Biologia Plantarum, 56(1): 64-70
http://dx.doi.org/10.1007/s10535-012-0017-2
Zhao H.X., Li Q., Zhou J., and Li G., 2010, The characteristics of low temperature tolerance during seed gemination of the ephemeral plant Lepidium apetalum (Cruciferae), Yunnan Zhiwu Yanjiu (Acta Botanica Yunnanica), 32(5): 448-454
Zhou Q., Li P. P., Zeng W. J., Li Y. H., Ge F. W., Yang N., Zhao H. X., 2016, De novo characterization of the seed transcriptome of Lepidium apetalum Willd, Zhongguo Shengwu Gongcheng Zazhi (China Biotechnology), 01: 1-11
. PDF(300KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Zhao Junjie
. Zhao Huixin
. Li Pingping
. Zeng Weijun
. Li Yanhong
. Ge Fengwei
. Zhu Changqing
. Lu Han
. Zhao Heping
Related articles
. Lepidium apetalum Willd.
. Seed Germination
. Germination Stagnation by low temperature
. Response protein
Tools
. Email to a friend
. Post a comment